Cutting-Edge Medical Advances: A Survey
Dr Purnoor Kaur
"To array a
man’s will against his sickness is the supreme art of medicine."
- Henry Ward Beecher
The pursuit of health and well-being has existed from the
beginning of time. Be it praying for elimination of wrath of Gods or viewing a
scorpion’s sting as purification, the denominator is unshaken. The twentieth
century has brought revolutionary changes in understanding of medicine and disease
processes and yet the twenty first century is hitting new heights. Alexander
Fleming and Penicillin had to wait for ten years but fortunately in recent
times medical research has gained momentum, measurability, and validation for
accuracy. The evolving challenges and dynamic database have made it possible to
pave a road for constant striving.
Health-related technology
The first robotic procedure was undertaken in 1992, three
decades down the line; today, there are three distinct types of surgical
robotic systems1 available. Active systems where most of the
work is done automatically (though the operating surgeon still has some degree
of control) and according to predefined procedures, semi-active systems where
the surgeon provides some input alongside the system's pre-programmed
components, and the formal master-slave architectures which do not have any
pre-programmed or autonomous parts for example laparoscopic surgical equipment
that mimic the surgeon's hands to complete a procedure.
Capsule cameras, also
called pill or swallowable cameras, are a novel medical device that has been
put to use in the diagnosis and monitoring of a wide range of digestive
problems. The initial prototype was released in 1998, and the FDA approved it
in 2001; the inventor is Gavriel Iddan. These cameras are tiny and
capsule-shaped such that they can be ingested and travel through the digestive
tract while capturing high-resolution photos of the body from the inside.
Capsule cameras have a variety of medical applications, but one of the most
common is in the diagnosis and follow-up care of inflammatory bowel diseases
(IBD), like Crohn's and ulcerative colitis. Inflammation and ulceration of the
digestive tract are hallmarks of these disorders, which can manifest as a variety
of unpleasant symptoms like nausea, vomiting, loss of appetite, and even weight
loss. Symptoms may be caused by inflammation or ulceration in the digestive
tract, which can be seen with capsule cameras. Other gastrointestinal diseases,
like gastrointestinal haemorrhage and small intestine anomalies, have also been
diagnosed and monitored with the help of capsule cameras. They have proven to
be especially helpful when using more conventional diagnostic procedures like
endoscopy or x-rays either isn't an option or isn't yielding the desired
results.
|
|
One of the biggest contributions of the pandemic is
catalysing the use of telemedicine, which allows patients to receive
medical care remotely via video or phone. This is especially useful for people
who live in rural areas or who have difficulty travelling to a medical
facility. However, Alaska has served as a model for the development of
telemedicine for decades, and radios were first employed in the 1920s, to give
medical advice to clinics on ships. Even, teleradiology has been used for at
least 60 years, and radiologists have promoted the Digital Imaging and
Communications in Medicine (DICOM) standard for transmitting and storing
data.
One of the earliest instances of hospital-based telemedicine
was in the late 1950s and early 1960s: A closed-circuit television link was
established between the Nebraska Psychiatric Institute and Norfolk State
Hospital for psychiatric consultations. (“3 The Evolution of Telehealth: Where
Have We Been and Where Are We ...”)
The next breakthrough is telesurgery, an emerging surgical
method, which uses wireless networking and robotic technologies to connect
distant clinicians and patients. The world’s first telesurgery was performed in
2001, which was performed by a surgical team in New York, USA, resulting in a
successful two-hour laparoscopic gallbladder removal of a female patient in a
hospital in Strasbourg, France.
|
|
3D printing
Since it first gained popularity in the early 2000s, 3D
printing has undergone substantial development in the recent decade. Its
applications have expanded beyond prosthetics to include customised implants,
surgical implements, and other surgical aids. In 2010, the FDA gave its first
green light to a business in Italy to use 3D printed orthopaedic implants. In
recent times, doctors in Belfast have used a 3D printed kidney (obtained from a
CT image of the patient) to pinpoint the precise location and size of a tumour.
The patient had a cyst on his kidney that may have developed into cancer, so
this served as valuable preoperative preparations in medical data that could
indicate the presence of a particular disease or condition, or to predict the
likelihood of a patient responding to a particular treatment.
The medical mirror
In 2011, a two-sided glass panel with a webcam and LCD
display was developed for use as a medical mirror. An automatic face tracker
can pick up on subtle changes in the blood vessels of the face caused by each
heartbeat.
The pain of needles may soon be forgone, researchers have
developed a novel idea called needle-free injection technology (NFIT),
which uses other means (shock waves, pressure, etc.) to administer medication.
In 2017, MIT debuted jet injections, one of the newest NFITs. A jet injection
shoots a thin stream of medicine (about the thickness of a human hair) under
high pressure through the skin at a controlled rate. It syncs with a mobile app
that records how the drug is working after each administration.
Gene editing:
What is Gene editing? It is the use of gene editing technologies,
such as CRISPR, to modify or repair genetic defects that cause diseases. The
invention of Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR)
in 2009, has the potential to revolutionise the way we treat many genetic
diseases. For example, gene editing could be used to repair or replace mutated
genes that are causing a genetic disease, or to introduce new genes that
provide a therapeutic benefit. Sickle cell diseases, cystic fibrosis, and even
some forms of cancer have been targeted by gene editing in animal models, and
clinical trials are currently underway to test its safety and effectiveness.
There are many technical and ethical challenges that need to be addressed
before it enters clinical practice.
In 2015, thanks to Juno, the new desktop DNA lab, the entire
human genome could be sequenced in about three hours. This method would not
only aid in locating the illness gene, but also in locating novel microbial
strains as soon as possible, allowing for the development of efficient
antibiotics to be used against them before they cause significant harm to human
life.
Artificial wombs
The development of artificial wombs is still in its infancy;
hence they cannot be used on people at this time. Before they may be employed
in clinical practice, several technological and ethical difficulties must be
overcome.
Artificial intelligence (AI)
Artificial intelligence (AI) is the ability of machines to
perform tasks that normally require human intelligence, such as learning,
decision making, and problem solving. (“Summary of the 2018 Department of
Defense Artificial Intelligence Strategy”)
|
|
Precision Medicine
Another trend that is likely to shape healthcare in the
future is the growing emphasis on personalised medicine. This is an approach to
healthcare that takes into account individual differences in people's genes,
environments, and lifestyles, in order to better diagnose, treat, and prevent
disease. By analysing large amounts of data, healthcare professionals will be
able to tailor treatments to the specific needs of an individual patient,
rather than using a one-size-fits-all approach.
It is also likely that healthcare in 2050 will be heavily
reliant on technology, with the use of electronic medical records,
telemedicine, and other digital tools becoming more widespread. These
technologies will allow healthcare professionals to access and analyse large
amounts of data in real time, improving the accuracy of diagnoses and the
effectiveness of treatment.
Overall, it is clear that healthcare in 2050 will be
significantly different from what it is today.
In the present times, we need not diagnose by solely reading
a pulse: we have an opportunity to explore genomic make up of individuals and
look forward to personalised or precision medicine. Undoubtedly, it is
one of the most exciting trends, which allows physicians to tailor treatment
plans to the specific needs of each individual patient.
Personalised medicine is based on the notion that because
individuals have complex and unique qualities at the molecular, physiological,
environmental exposure, and behavioural levels, they may require interventions
tailored to their nuanced and unique characteristics for diseases. Emerging
evidence has revealed significant inter-individual variation in the effects of
disease processes, as well as the mechanisms and factors that contribute to
them. This has generated concerns regarding how much inter-individual variation
should influence decisions about the best method to treat, monitor, or prevent
a disease for an individual.
There are several hurdles involved with individualised
medicines, particularly acquiring authorisation for routine use from various
regulatory organisations. Furthermore, there have been numerous challenges
related with the widespread acceptance of personalised medicines among various
health care stakeholders, including physicians, health care executives, insurance
companies, and, ultimately, patients.
Origin story
Archibald Garrod, an English physician, began examining disorders that would eventually be known as inborn errors of metabolism more than a century ago. Garrod researched a variety of rare disorders with obvious phenotypic characteristics, such as alkaptonuria, albinism, cystinuria, and pentosuria. He concluded that alkaptonuria was caused by a specific altered course of metabolism in affected persons, which was later confirmed to be correct. In addition, when considering other rare diseases such as alkaptonuria, Garrod argued that "...the thought naturally presents itself that these [conditions] are merely extreme examples of variation in chemical behaviour that are probably everywhere present in minor degrees and that just as no two individuals of a species are absolutely identical in bodily structure, neither are their chemical processes carried out on exactly the same lines." This hints at his notion that, at least in terms of metabolism, humans vary greatly, and that these changes in metabolism could help explain overt phenotypic differences between individuals, such as their various susceptibilities to diseases and the ways in which they display diseases.
Although personalised medicine has its roots in genetic
studies, it is commonly understood that additional factors (environmental
exposures, developmental phenomena, epigenetic modifications, and behaviours)
must also be considered when identifying the best strategy to treat an
individual patient.
Laura H. Goetz M.D. and Nicholas J. Schork Ph.D.
Fertility and Sterility, 2018-06-01, Volume 109, Issue 6,
Pages 952-963, Copyright © 2018 American Society for Reproductive Medicine
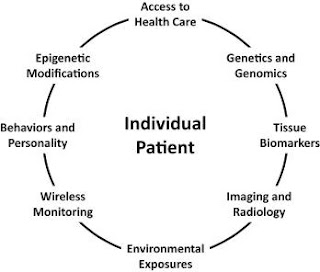
Access to health care is critical because some people may be unable to access expertise and technologies owing to geographical or economic restrictions. As a result, interventions for those persons may need to be designed with this in mind. Inherited genetic information can only be predictive or diagnostic. However, somatic DNA alterations can provide valuable information about disease processes. Tissue biomarkers (e.g., routine blood-based clinical chemistry panels), imaging and radiology exams, and data obtained routinely via wireless monitors are all valuable for detecting changes in health status. Environmental exposures and behaviours can have an impact on intervention outcomes and have a high intra-individual variability. Epigenetic events, which remodel gene function because of exposures and developmental or stochastic phenomena, should be examined as markers of a change in health status.
Although straightforward in theory, the practical challenges
associated with acquiring more information about a patient and conducting an
empirical evaluation of a personalised remedy can be overwhelming. Questions
such as how to know if a chosen intervention works without meticulous patient
follow-up data, how to know if a patient is satisfied with what they are
experiencing with the intervention, and how to assess the difference between
other interventions that could have been chosen and the chosen personalised
intervention would all need to be addressed.
Examples of individualised medicine in the modern era
Drugs such as warfarin, PQ, and imatinib that appear to only
work, or work without side effects, when a patient has a specific genetic
profile have sparked intense interest in identifying factors, such as genetic
variants, which influence an individual patient's response to a variety of
drugs and interventions. This interest in developing tailored medicines to
treat diseases has widened to include personalised disease surveillance, such
as early detection techniques and personalised disease preventive strategies.
After detailing a few more recent examples of tailored medicines, we briefly
highlight a few very recent examples of this activity.
Immunotherapies, a new group of cancer treatments, are
another example. Immunotherapies come in many different forms, but they all try
to get a person's own immune system to fight cancer. This type of immunotherapy
basically works by taking cells from a patient that control their immune
reactions and changing them so they can find and attack the neo-antigens found
in the patient's tumour. Then, these changed cells are put back into the
patient's body, where they attack tumour cells and send out signals for
neo-antigens.
Early Detection Strategies
If a person is prone to getting sick that person should be
closely watched. Epidemiologic data and population surveys are used to make
population thresholds. For example, a cholesterol level of more than 200 is a
sign of a higher risk of heart disease, and a systolic blood pressure of more
than 140 is a sign of hypertension, stroke risk, or heart disease. The past
values of a measure for a person are used to make a personal threshold, which
is then used to predict how different the future values of that measure may be
for that person. Significant changes from historical or average legacy values
are seen as a sign of a change in health status, regardless of whether the new
values exceed a population threshold
Individualizing Disease Prevention
Even though it is well known in the scientific community
that genetic information can be used to create personalised ways to prevent disease,
this is not yet widely used in clinical practice. There are many notable
examples of how genetic information can be used to lower the risk of getting a
disease and the problems that come with current treatments and screening
methods.
References
- Medicine I of, Services B on HC. The Role of Telehealth in an
Evolving Health Care Environment: Workshop Summary. National Academies
Press; 2012.
- Laura H. Goetz M.D. and Nicholas J. Schork Ph.D. Fertility and
Sterility, 2018-06-01, Volume 109, Issue 6, Pages 952-963
- Summary of the 2018 Department of Defense Artificial Intelligence
Strategy
- Is Machine Learning Hard? A Guide to Getting Started,
https://learnsic.com/blog/is-machine-learning-hard-a-guide-to-getting-started.
- Henry Ward Beecher - To array a man's will against his... -
BrainyQuote, https://www.brainyquote.com/quotes/henry_ward_beecher_118242.




.gif)
.png)


.png)

.png)
.png)
.png)
.png)
.png)
Comments
Post a Comment
Share your response